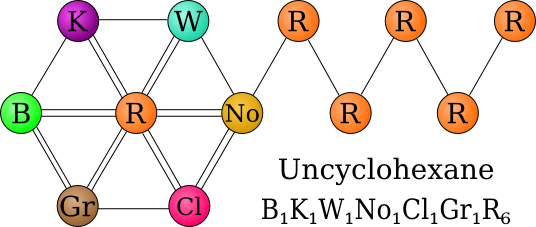
Uncyclohexane
Uncyclohexane is the name of a covalent compound that constitutes most of Uncyclopedia's mass. It is composed of the elements Ballmerate, Kanyite, Wildium, Norrium, Clichane, Grue, and Randium.
Toxic in even small doses and prone to spontaneous energy releases, Uncyclohexane is a dangerous substance that is banned in many countries.[1]
Geometry[edit]
Uncyclohexane is a hexagonal molecule composed of several mildly toxic atoms bound together by single and double bonds. The chemicals on the edges (with the exception of Grue,[2] Clichane,[3] and Norrium[4]) are rendered inert by covalent bonding with a single Randium atom in the center.
Uncyclohexane's side chain (composed of five Randium atoms[5]) is very unstable and prone to spontaneous energy release. Randium atoms are three electrons away from filling their octet, so they bond with one another to fill two of those spaces.[6] The result is a highly reactive and volatile substance that is able to asplode nearby molecules and spontaneously detonate kitten brains . Ballmerate, although highly reactive in its isolated form, is inert in Uncyclohexane, as are Kanyite and Wildium.
Uncyclohexane is classified as a Type 4 Dangerous Material by the United States Chemical Bureau for its radioactivity, reactivity, toxicity, and its tendency for quantum distortion.
Origin and catalysis[edit]
With its inherent danger and uselessness, one may wonder why Uncyclohexane is so prevalent in Uncyclopedia. The answer lies in the many varieties of cellular enzymes that perform basic tasks on Uncyclopedia.
Anonase, a very basic enzyme, creates Uncyclohexane from extraencyclopediar materials. Anonase then proceeds to waste ATP transporting the Uncyclohexane inside the encyclopedia. Anonase is normally incapable of performing any other task besides creation and import of Uncyclogen, although on rare occasions anonase has been seen catalyzing Uncyclohexane and even importing pure Uncyclogen at least once.
Userase, an enzyme superior to anonase, performs several functions. Userase can create and import Uncyclohexane, albeit less frequently than anonase. Userase also imports pure Uncyclogen on rare occasions. However, userase's main role in the encyclopedia is to catalyze the transformation of Uncyclohexane into Uncylogen (a far superior substance).
Adminase, the highest level of encyclopedia enzyme, can perform any function. Adminase is unable to create Uncyclohexane at all, instead synthesizing pure Uncyclogen. Adminase is able to work at a much faster pace than either anonase or userase, and is less prone to errors. Adminase can also allosterically inhibit anonase or userase to keep them from running rampant.
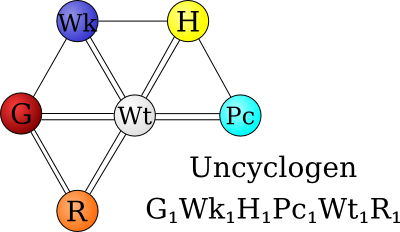
Uncyclogen[edit]
As dangerous and useless as Uncyclohexane is, it serves one vital purpose. That purpose is to provide the basis for synthesis of a different molecule, Uncyclogen.
Uncyclogen is far more stable than Uncyclohexane and presents no danger to any substance, as nearly all of its components are nonreactive.[7]
It is composed of the elements Gramane, Wikiate, Humercury, Pictite, Wittium, and Randium.[8]
It is arranged vaguely like Uncyclohexane, but is entirely different in chemical nature. Although it shares a characteristic with Uncyclohexane in that they are both bound together by double bonds with a central atom, Uncyclogen only has five outer atoms and lacks a side chain. Uncyclogen is also very strong and can bind with other Uncyclogen molecules to form a strong lattice-like structure. There are only three atoms in Uncyclogen which do not have full valence shells: Wikiate, Pictite, and Randium.[9] Pictite is unique among these three free atoms in that two Uncyclogen molecules can share one Pictite atom.
Uncyclogen constitutes a small portion of Uncyclopedia. It was classified as a Type 5 Integral Polymer by the USCB.
Notes[edit]
- ↑ Except Switzerland, where pretty much anything is legal.
- ↑ Although rendered inert by the covalent bonding, Grue's full octet shell destablises the nucleus, thereby making Uncyclohexane radioactive.
- ↑ Clichane's covalent bonds somehow trigger a quantum reaction in which it will distort the space-time continuum so that slowly, all atoms that are near for a long enough time will also turn to Clichane.
- ↑ Norrium is an exceptionally violent element, whose lone valence electron is fortunately provided by Randium. It should be noted that, when isolated, Norrium is capable of single-handedly destroying both covalent and ionic bonds.
- ↑ Exceptions have been observed, the most dramatic being an Uncyclohexane molecule with an enormous 86-Randium side chain
- ↑ A "trussed" structure would be impossible because for unknown reasons, a nuclear explosion is triggered when one Randium binds to three others.
- ↑ With the exception of Wikiate, Pictite, and Randium.
- ↑ Randium, coincidentally, is the only element that is present in both Uncyclogen and Uncyclohexane.
- ↑ Randium in Uncyclogen is dramatically less reactive than Randium in Uncyclohexane.