Hydrocarbon

“putting the HOMO in homologous series”
The term Hydrocarbon comprises many groups and series within the field of chemistry and as such is a many-faceted area predominately involved with blowing things up - but this time with many useful applications and functions, promise.
Simply put, hydrocarbons are chemical species containing soot and inflamable air. This unlikely combo has stood the test of time - becoming a popular group of chemicals overtaking halides as 'Homologous series of the year' every year since 2001. In concordance with those bastards at the IUPAC, this article will use 'C' to represent coal dust and 'H' to represent blimp-gas.
Alkanes
Alkanes are often thought of as the simplest of the hydrocarbons, however to the informed, quite the opposite is true. Hidden beneath their basic appearance lies increasingly subtle layers of intrigues- ranging from the painfully mundane (picking up their kids from the creche ect) to overwhelmingly complex (Reviewing the top ten sonic slashfics on FanFiction.net).They have the general formula where 'n' is the number of grains of soot put into the witch's pot before formulating the alkane.This general formula however doesn't account for side chains formed when too many farts get near the conical test tube thingy whatever device.The simplest alkane is in fact Fartium which consists of but one soot granule beset on all sides by pockets of common laboratory runoff. You may have already deduced that this means everybody who has ever farted has ingested coal at one point in their lives (don't lie this is an empirical FACT you creepy charcoal junkies).
Alkenes
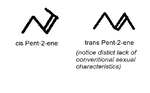
Alkenes like to think that they're more sophisticated than those backwater Alkanes just because they possess a carbon double bond whatever the fuck that means (seriously why would they put that in this textbook what does it mean?). Ironically this upper-class quirk leaves it with a simpler general formula which it cleverly employs in order to get out of paying tax.This formula is , which once again doesn't account for bonus fart chains or even more double bonds from the carbons getting extra cuddly.Unlike normal bonds, double bonds are always trying to trick chemistry students by coming in multiple forms namely 'cis' and 'trans' (pictured). Surprisingly trans isomers are less kinky than cis isomers (which will even do that very secret and bad thing with you) meaning that trans-fats are scientifically the least sexy of all molecules- even less than m̶e̶t̶h̶a̶ fartium.
Long hydrocarbon chains
Hydrocarbons can exist at vast lengths, some even visible from space, such chains are currently being spread around the earth and used to squeeze minerals from deep beneath the surface. The program was developed by a team of chemists under supervision of Richard 'Dayum' Feynman after observing an oily teenager using a hydrocarbon chain to burst a zit. The team are currently under consideration for a posthumous Nobel Prize although further investigation has linked the nomination to an oil company conglomerate. More complex long hydrocarbon chains are synthesized via polymerization (pictured) a process in which lots of small baby molecules are grouped together to become mighty fibers such as 'coats' and 'hats' ect.
The nature of the polymer depends on the functional groups that have managed to cling on to the side of the chain, often found in nature it is no coincidence many of these larger complex molecules resemble plants and animals - and in addition many of these organisms traits are derived straight from the properties of the functional groups withing the chain.
functional groups
| Symbol | Name | Function |
|---|---|---|
| CH, CHCH | Animal shapes | Giving animals floppy limbs and apendages |
| OH | Booze-stuffs | Messing up people's brains, smelling bad |
| COOH | Vinegar and lemon juice | Rubbing in wounds, not smelling |
| NH | Amines | Making drugs, smelling bad |
| C=O | Aldehydes and Ketones | Preserving dead mice, smelling bad |
| C=OC(OH) | Smelloids | Smelling good |
| COOL | Sweetages | Making you look totally rad |
More complex series
Hydrocarbons form the basis of several useful and fun chemicals found all over the home and work-place. These include (but are not limited to) fats, soaps, drugs, alcohols, esters, solvents, acids and with enough soot even amino acids and proteins. This means that a proper knowledge of hydrocarbons can allow you to provide a drink laced with depressants, lean in close with an overpowering aroma, burn away at your inhibitions, dissolve your consciousness, raise your heart rate, dilute your pupils, bring you to an incredible high and then leaved you cleaned out, loins frothing for more - much like Your mom does.
See also
| Featured version: 8 March 2015 | |
| This article has been featured on the main page. — You can vote for or nominate your favourite articles at Uncyclopedia:VFH. | |